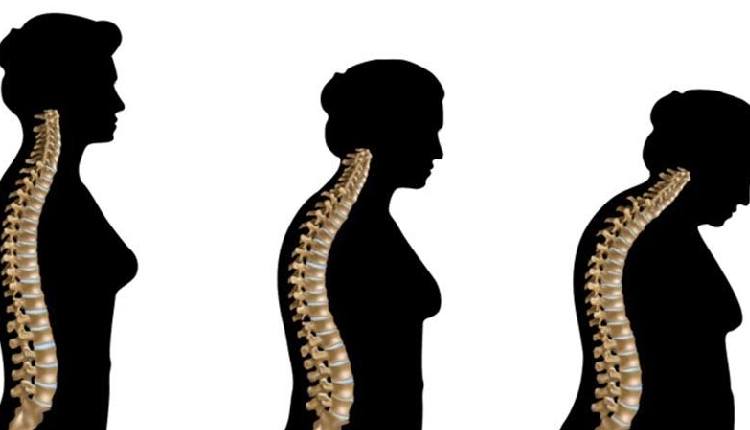
Seoul: Celltrion, a major South Korean biopharmaceutical firm, said on Tuesday its two new bio-similars for bone disease treatment have obtained approval from the United States.
The U.S. Food and Drug Administration (FDA) approved Celltrion’s Stoboclo and Osenvelt, biosimilar drugs to Prolia and Xgeva, respectively, in the form of subcutaneous formulations for sales in the US market, the company said in a press release, Yonhap news agency reported.
The global market for Prolia and Xgeva was estimated to have reached a combined 9.2 trillion won ($6.6 billion) last year, it said.
The U.S. accounted for 6.15 trillion won, or 67 percent, of the two original drugs’ sales last year.
Last month, Celltrion obtained approval from the FDA for U.S. sale of Avtozma, an autoimmune disease biosimilar to Actemra, in both intravenous and subcutaneous formulations.
The Korean drugmaker aims to commercialise 22 biosimilar products by 2030, up from the current 11.
Meanwhile, Celltrion’s fourth-quarter net profit skyrocketed from a year earlier on robust sales of bio-similar medicines and the merger with its health care affiliate.
Net profit for the three months ended on Dec. 31 soared to 235.6 billion won ($164.7 million) from 453 million won during the same period of 2023, the company said in a regulatory filing.
“The net result got a boost from sharply increased sales of our biosimilar products, including Remsima, Truxima and Herzuma, in advanced markets and hefty cost reduction following the merger with Celltrion Healthcare in late 2023,” a company spokesperson said.
Remsima is a treatment for Crohn’s disease and other autoimmune diseases, while Truxima and Herzuma are for anti-cancer treatment.
Operating profit also jumped over 10-fold to 196.4 billion won in the fourth quarter from 18.4 billion won a year ago.
Sales nearly tripled to 1.06 trillion won from 382.6 billion won over the same period.
(IANS)