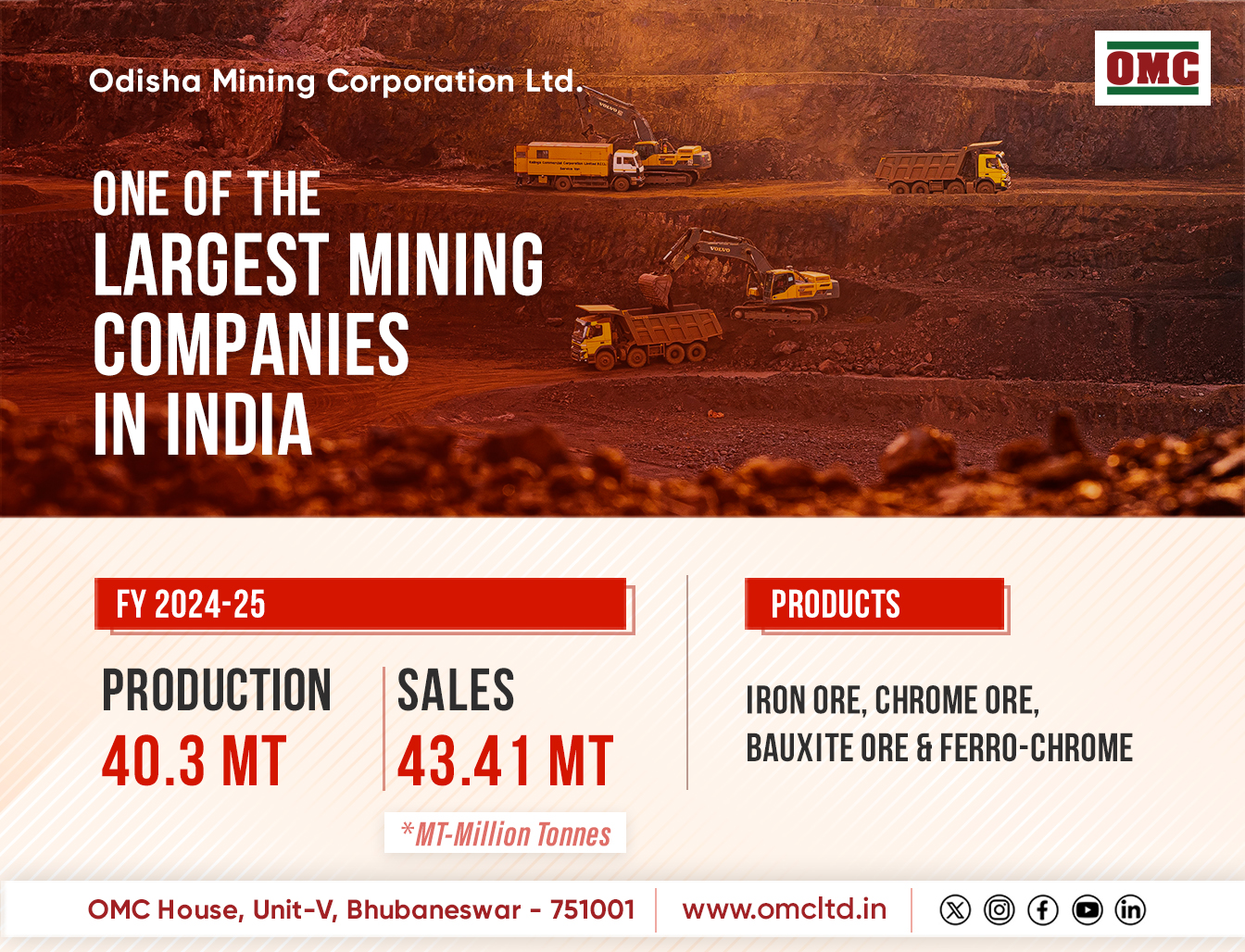
Bharuch: The anticipated bulk drug park in Jambusar is set to witness accelerated progress, with the Gujarat government gearing up to initiate the tender process for the park’s communal facilities and the allotment of land to companies in the near future.
Spanning a sprawling 2,000 acres, this upcoming park is poised to play a pivotal role in expediting the domestic production of critical Key Starting Materials (KSMs), Active Pharmaceutical Ingredients (API), and pharmaceutical intermediates — commodities that are currently imported from China. The Indian Drug Manufacturers’ Association (IDMA) envisions the park as a potential home for around 400 companies, attracting investments exceeding Rs 8,000 crore.
Viranchi Shah, President of IDMA, underscored Gujarat’s status as a pharmaceutical hub that significantly contributes to both the nation’s exports and production capabilities. In a bid to reduce reliance on imported bulk drugs, the central government proposed the establishment of three bulk drug parks across India, one of which is slated for Jambusar.
The collective facilities within the park, including nitrogen utilities, a Common Effluent Treatment Plant (CETP), solvent recovery units, training centers, testing laboratories, and research spaces, are anticipated to become operational by the conclusion of the fiscal year.
The bulk drug park is anticipated to attract approximately 400 diverse companies engaged in the manufacturing of a wide array of pharmaceutical products. By leveraging the shared facilities provided by the government, production costs can be streamlined, enhancing competitiveness against the pricing strategies of Chinese counterparts.
Despite witnessing a notable 5.1 per cent growth in pharmaceutical exports during the initial quarter of the 2023–24 fiscal year, the industry’s reliance on imported raw materials has augmented. Notably, imports of Active Pharmaceutical Ingredients (APIs) surged from $2,110 million in 2015-16 to $3,180 million in 2022-23, with a significant portion sourced from China.
(IANS)