New Delhi: Scientists from Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR) Bengaluru, an autonomous institution under the Department of Science & Technology (DST), on Thursday provided groundbreaking insights into a new class of materials for energy harvesting and power generation.
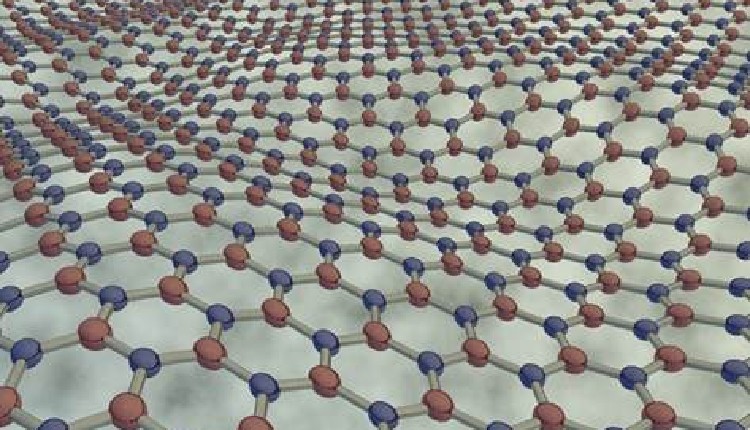
Their work unraveled the electronic mechanisms governing chemical bonding of new class of materials called incipient metals with metavalent bonding (MVB) within a single 2D layer of Group IV “chalcogenides” that can boost energy harvesting and power generation.
Chalcogenides can transition reversibly between amorphous and crystalline phases in response to changes in temperature, pressure or electrical fields.
The study by Professor Umesh Waghmare from Theoretical Sciences Unit at JNCASR, explored the possibility of introducing the metavalent bonding (MVB) within a single 2D layer of Group IV chalcogenides, investigating its mechanisms and the resulting consequences on material properties.
“These materials, termed incipient metals, possess a combination of properties that defy conventional understanding. They exhibit electrical conductivity akin to metals, high thermoelectric efficiency characteristic of semiconductors, and unusually low thermal conductivity, creating a triad of properties that cannot be explained by traditional chemical bonding concepts,” explained Professor Waghmare.
The study, published in Angewandte Chemie International Edition and supported by JC Bose National Fellowship of the Science and Engineering Research Board-DST and JNCASR research fellowship, provides a first-principle theoretical analysis focusing on the bonding nature within five different 2D lattices of Group IV chalcogenides.
This category includes compounds which exhibit remarkable properties, transitioning reversibly from a glassy amorphous structure to a crystalline form in less than 100 nanoseconds when subjected to heating or cooling.
Driven by an idea presented by Professor CNR Rao, the study aimed to unravel the electronic mechanisms governing the chemical bonding in these materials.
The findings, which took nearly two years of theoretical and computational work, have shed light on the unique properties of these materials, challenging conventional chemical bonding ideas.
(IANS)