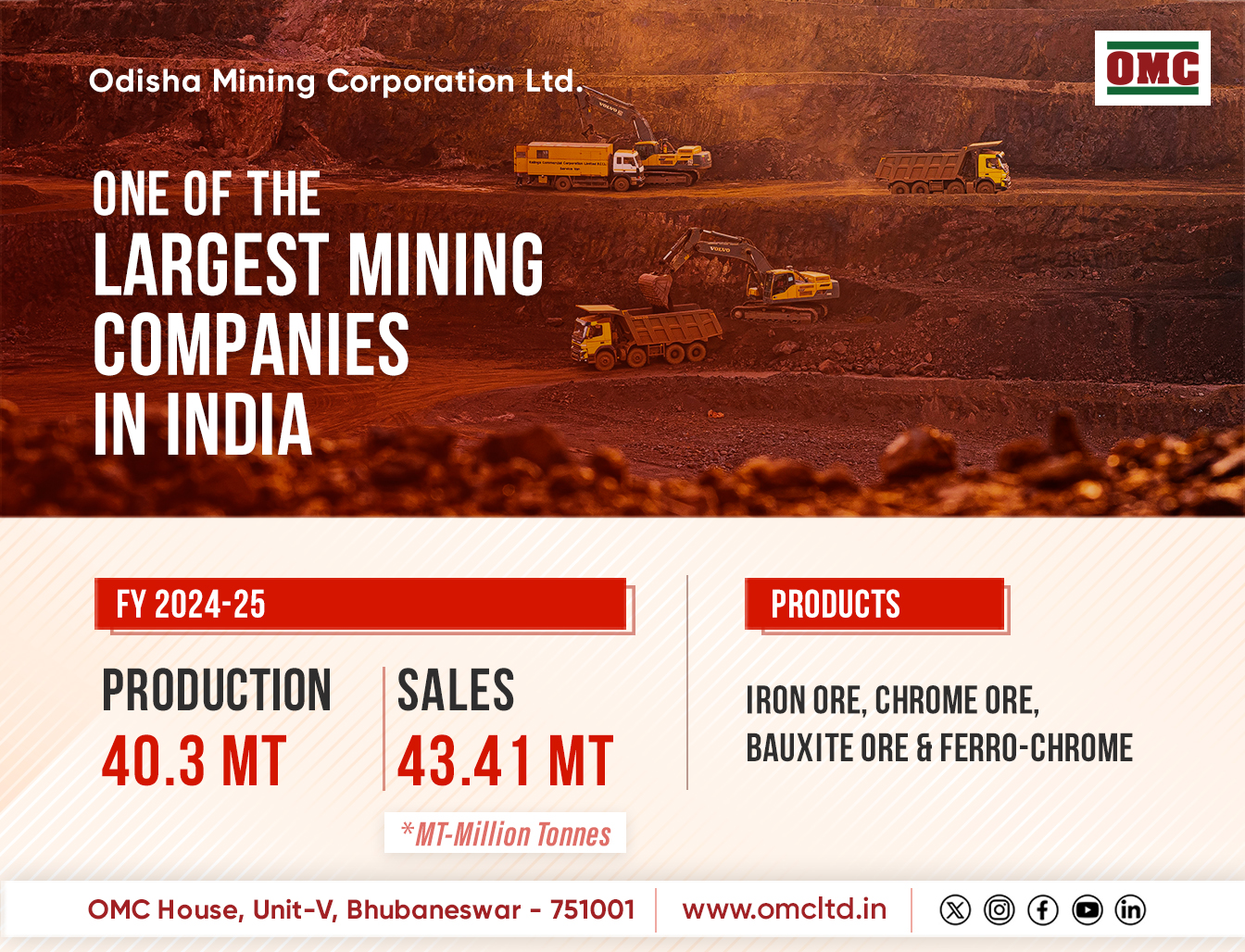
Washington: In a first, the US Food and Drug Administration (FDA) has granted full approval to a drug to slow down disease progression in adult patients with Alzheimer’s disease.
Alzheimer’s disease is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills and eventually, the ability to carry out simple tasks.
While the specific causes of Alzheimer’s are not fully known, it is characterised by changes in the brain — including the formation of amyloid beta plaques and neurofibrillary, or tau, tangles — that result in loss of neurons and their connections.
Developed by drugmakers Eisai and Biogen, Alzheimer’s drug lecanemab, known by the brand name Leqembi, is to be administered intravenously every two weeks and will be available for people diagnosed as having early-stage Alzheimer’s and for those with a pre-Alzheimer’s condition called mild cognitive impairment.
Leqembi, the amyloid beta-directed antibody, works by reducing amyloid plaques that form in the brain — a defining pathophysiological feature of the disease.
While the drug is not a cure for Alzheimer’s, it has shown to reduce the rate of disease progression and to slow cognitive and functional decline in adults with Alzheimer’s disease.
“Today’s action is the first verification that a drug targeting the underlying disease process of Alzheimer’s disease has shown clinical benefit in this devastating disease,” said Teresa Buracchio, acting director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research.
The drug was granted an accelerated approval earlier in January, based on one mid-stage study in 800 people with early signs of Alzheimer’s who were still able to live independently or with minimal assistance.
The final approval was based on a Phase 3 randomised, controlled clinical trial including 1,795 patients with Alzheimer’s disease, where Leqembi demonstrated a statistically significant slow decline by about five months over a period of 18 months for those patients.
“This confirmatory study verified that it is a safe and effective treatment for patients with Alzheimer’s disease,” Buracchio said.
However, it doesn’t improve patients’ memories or cognitive abilities, and also does not stop the disease from getting worse.
Moreover, the FDA said that the drug can cause swelling or bleeding in the brain that is often mild or moderate and resolves on its own but can be serious and in very rare cases can be fatal.
To prevent this, the regulatory body has also asked the companies to issue a “black-box warning” — the most urgent level — on the drug’s label, saying that the medication can cause “serious and life-threatening events”.
Further, the FDA said that patients with higher risk include those on blood thinners, those who have had more than four microscopic bleeds in the brain and those with an Alzheimer’s-linked gene mutation called APOE4 — especially if they have two copies of the mutation.
The drug is reportedly expected to be priced at about $26,500 for a typical year’s worth of treatment, without insurance.
(IANS)