New Delhi: Indian drug maker Zydus Lifesciences on Saturday announced receiving final approval from the US Food and Drug Administration (USFDA) to market a generic medication for arthritis and other conditions.
The company received approval to market Dexamethasone Tablets USP, 1 mg, in the US.
Dexamethasone is used to treat conditions such as arthritis, blood/hormone disorders, allergic reactions, skin diseases, eye problems, breathing problems, bowel disorders, cancer and immune system disorders.
The product will be manufactured at the group’s formulation manufacturing facility at Baddi, Himachal Pradesh, the drug maker said.
According to the data by the global provider of advanced analytics, technology solutions IQVIA, Dexamethasone tablets had an annual sales of $1.8 million in the US.
Earlier in the week, Zydus Lifesciences received final approval from the USFDA to market Dapsone Gel, 7.5 per cent.
Dapsone Gel is used to treat acne and will be manufactured at the group’s topical manufacturing facility at Changodar in Gujarat’s Ahmedabad.
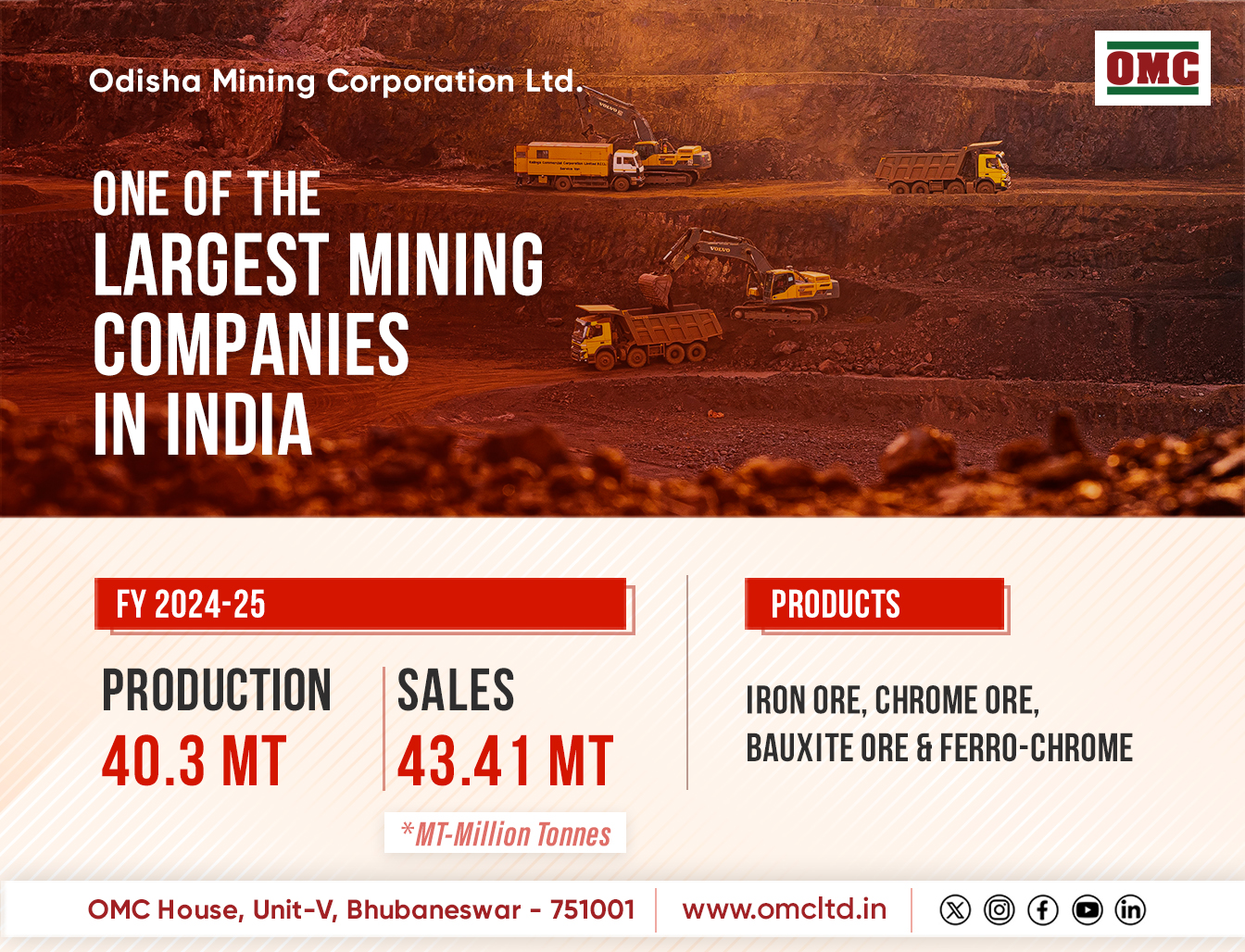
As of December last year, the group has 395 approvals and has so far filed more than 460 Abbreviated New Drug Applications (ANDAs) since the commencement of the filing process in FY 2003-04, the company mentioned.
(IANS)