New Delhi: Researchers at the S. N. Bose National Centre for Basic Sciences, Kolkata, an autonomous institute under the Department of Science and Technology (DST), have found an efficient, less energy-intensive, and environmentally friendly way to synthesise hydrogen peroxide — a chemical that is crucial to the industry for disinfection, paper bleaching.
Hydrogen peroxide (H2O2) is a versatile oxidising agent that is widely used in environmental disinfection, chemical synthesis, paper bleaching, and fuel cells.
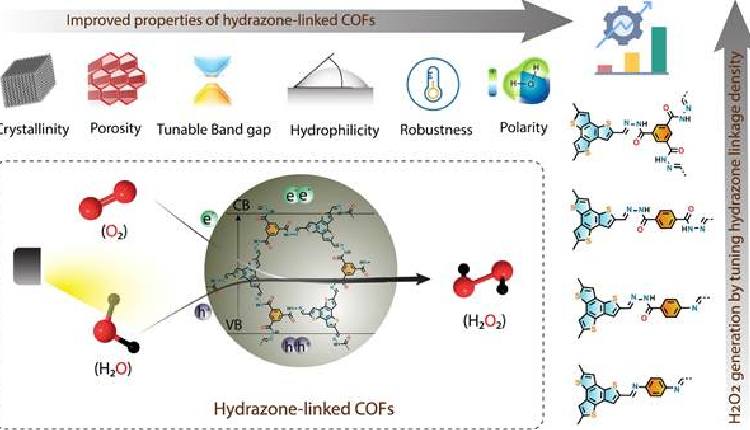
The team designed and prepared a series of covalent organic frameworks (COFs) having good water affinity through careful control of the hydrazone linkage density and studied their effect on the photocatalytic performance for H2O2 generation. COFs are a new class of porous and ordered polymers with modifiable catalytic sites and light-harvesting properties in the visible range. It has emerged as a promising photocatalysts.
Currently, the growth in the H2O2 market is driven by the increasing awareness of disinfection, the rise in the number of surgeries, and the prevalence of hospital-acquired infections. More than 95 per cent of H2O2 is produced industrially using the anthraquinone oxidation process — which is very energy intensive, expensive, and produces many hazardous chemicals as by-products.
Scientists have therefore been looking for an environmentally friendly and economical strategy to produce H2O2 from renewable resources with minimal environmental impact.
The study observed that the hydrazone-linked COFs provide abundant docking sites for water and oxygen, thereby promoting water oxidation reaction (WOR) and oxygen reduction reaction (ORR) – two main pathways for photocatalytic H2O2 generation.
As a result, the hydrazone-linked COF exhibited exceptional photocatalytic H2O2 production without external sacrificial electron donors when irradiated with a 40 W blue LED.
Interestingly, “a significant amount of H2O2 was also produced under sunlight irradiation, which outperforms most organic photocatalysts under similar conditions, thus demonstrating a clean and sustainable pathway,” said the team.
They showed that as-synthesized hydrazone-linked COFs can generate a significant amount of H2O2 using an aqueous benzyl alcohol solution (water: benzyl alcohol = 90:10).
The method also prevented the degradation of H2O2. The “strategy of using a mixture of water-benzyl alcohol solution will be helpful in developing a continuous flow reactor for the sustainable production of H2O2 and will reveal a laboratory-to-industry technology transfer for the benefit of mankind,” noted the researchers.
(IANS)