Bhubaneswar: Health & Family Welfare (H&FW) Department of the Odisha Government is committed to provide accessible and affordable quality health care to the people of the State.
In view of the goals of universal health coverage and in order to reduce the out of pocket expenditure towards Diagnostics at Public Health Facilities, “Free Diagnostic Services – Nidaan” was announced by the Chief Minister on 19th December 2017 and was launched on 1st January 2018.
The main objectives of Nidaan is to ensure assured diagnostic services in public health facilities as per its level of care so as to reduce the out of pocket spending (OOPS) towards diagnostics through the strengthening of public health facilities; and to establish collaboration with private service providers to ensure high-end Pathology tests, Tele-radiology (X-Ray), MRI & CT-Scan services at public health facilities.
Keeping in view the healthcare needs of the population and to augment the diagnostic services under “Nidaan”, the H&FW Department through a notification issued today has expanded the range of assured diagnosis services (Essential Diagnostic List) for different categories of facilities as per its level with following stipulations:
i. The `Nidaan’ – Free Diagnostic Services’ is assured for all population of the State attending public health facilities both inpatient and outpatient health care.
ii. Under “Nidaan” the identified diagnostic services have been provided to in-house/outsourced mode in all public health facilities from Sub Centres to Medical Colleges & Hospitals on free of cost to all patients from 01.01.2018.
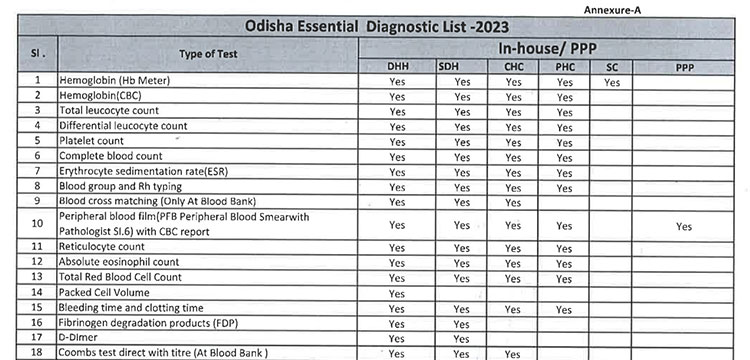
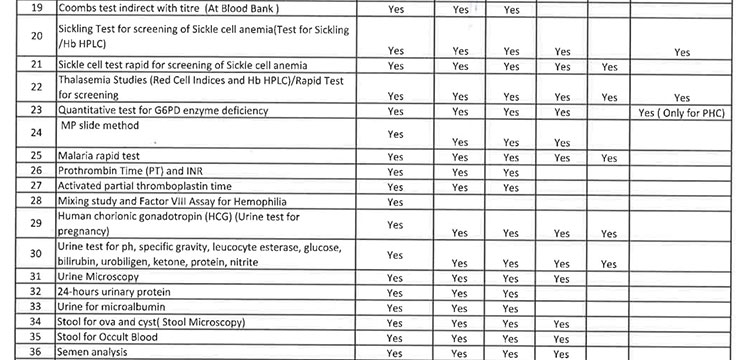
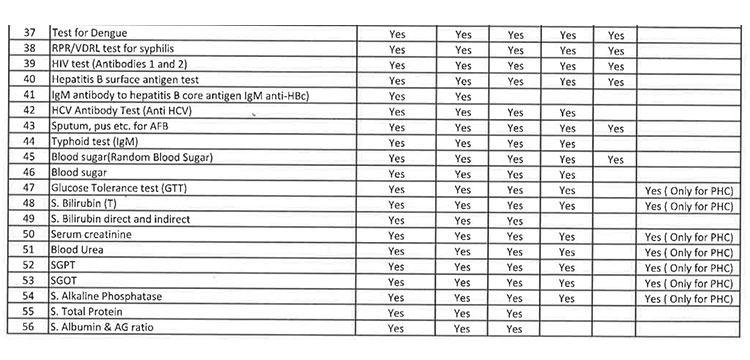
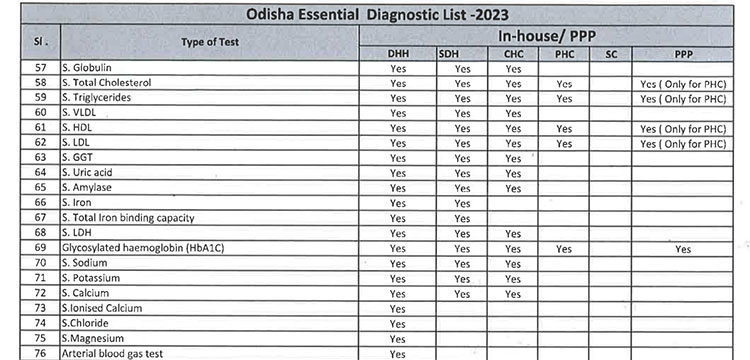
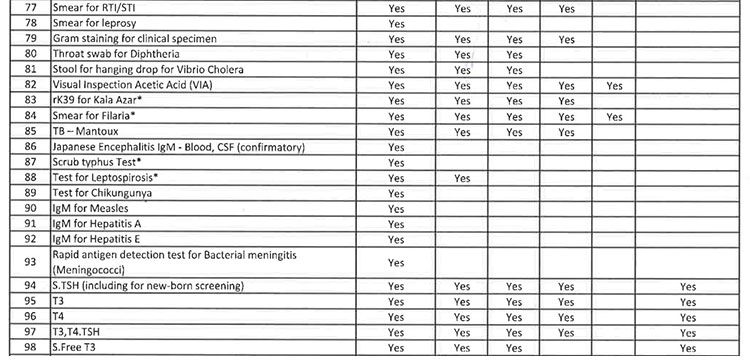
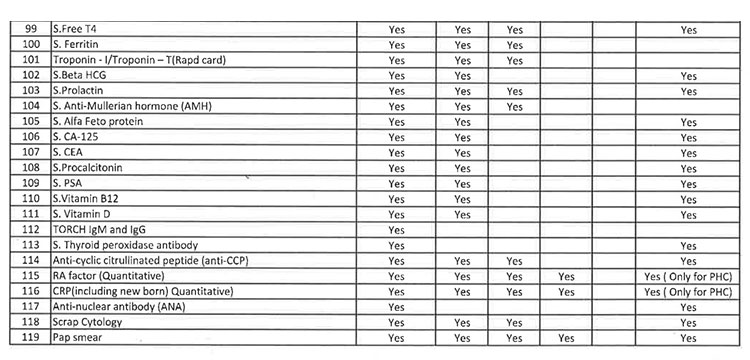
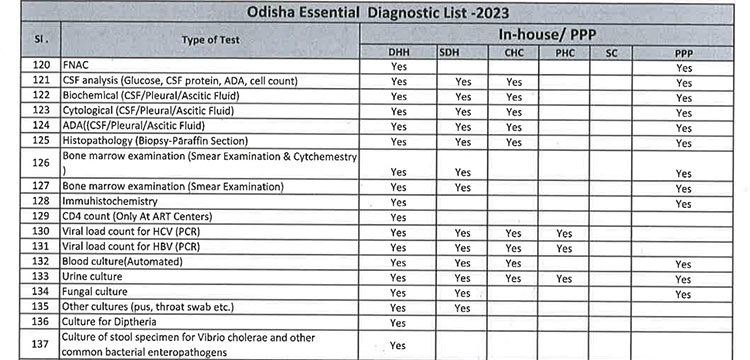
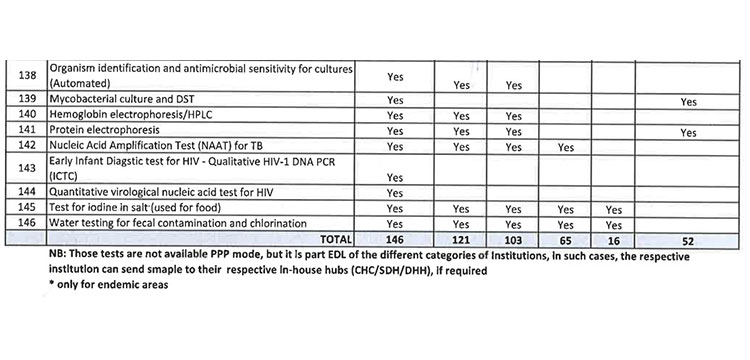
iii. As per the revised Essential Diagnostic List, 16 types of test at Sub Centres (SCs), 65 types of test at Primary Health Centre (PHC-N), 103 types of test at Community Health Centres, 121 types of test at Sub Divisional Hospitals and 145 types of test at District Headquarter Hospitals will be assured to all patients. However, all Government Medical College & Hospitals would continue to provide 221 types of test as per the previous order. However, the Essential Diagnostic List may be modified keeping in view the priority of the State and the disease burden from time to time. The essential diagnostic list for different categories of facilities is attached at Annexure-A.
iv. For General Pathology Services, kit/ POC based test will be done at Sub Centres and PHC(N) / OH without DMCs and through integrated lab as well as through Kit-based test at PHCs, CHCs, SDHs, DHHs & other bedded hospitals. However, it will be provided at different service delivery locations at all Government Medical College & Hospitals.
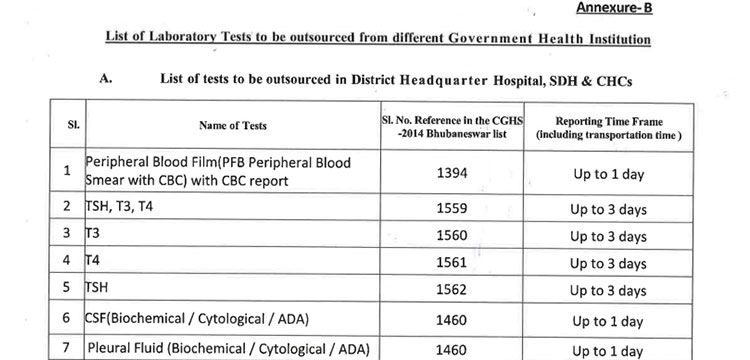
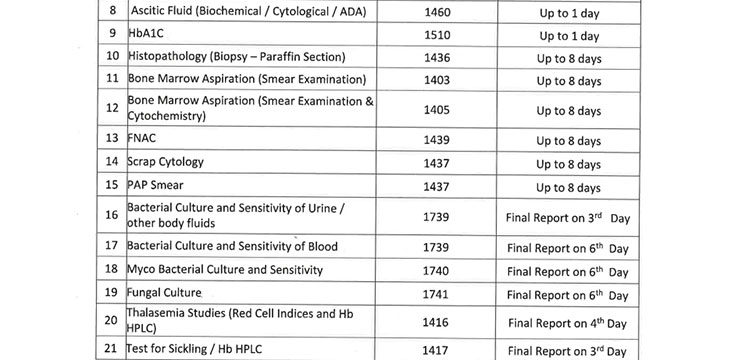
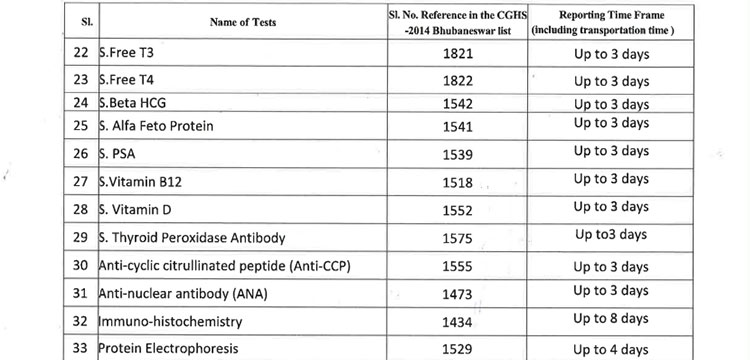
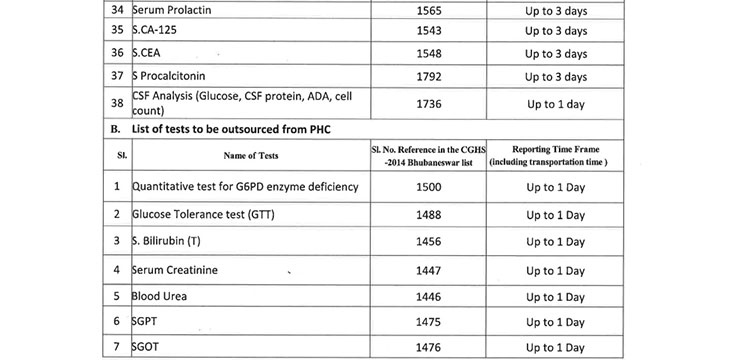
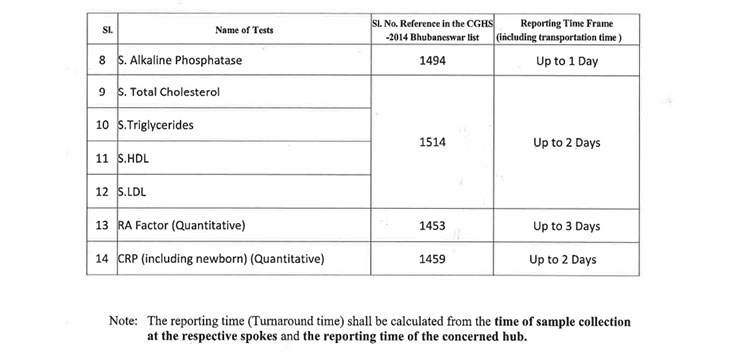
v. 52(38 upto U/CHC level and 14 for U/PHC level) types of Pathology tests will be provided through outsourced agency selected through Gem tender. Agency will establish sample collection centers at all Block CHCs/UCHCs, SDHs & DHHs and provide services as per the terms and conditions of the MoU conditionalities. The list of high-end pathological tests & defined time frame for report is attached at Annexure-B.
vi. Provision should be made to ensure quality of tests which are dependable, accurate, timely, easily accessible and affordable to the people.
vii. All essential diagnostic services will be provided only to the patients referred from the public health facilities in the State.
viii. No reimbursement will be given to patients for tests prescribed in public facilities but conducted in private centers.
ix. List of assured diagnostic services will be displayed in all public facilities in prominent places for public information and awareness.
x. As the identified services will be provided free of cost hence, no user fees will be collected from patients towards assured diagnostic services as per list at all public health facilities including Government Medical Colleges & Hospitals which was waived w.e.f 01.01.2018.
xi. The loss of user fees collection will be compensated through release of commensurate institutional developmental cost to all public health facilities.
xii. All types of tests should be provided based on existing Standard Treatment Guidelines and Standard Operating Procedures (SOP) available for different diseases or conditions and to be provided from time to time. Moreover, all types of investigations need to be carried out for the right reasons, on the right specimens, at the right times and right places and the result need to be interpreted and used appropriately.
xiii. Quality of services will be ensured through appropriate external and internal quality assurance and quality control measures, as prescribed in National Quality Assurance Standards (NQAS) and other statutory provision like AERB etc.
xiv. The laboratory and bio-medical safety guidelines must be followed strictly for safety of both patients and service providers.
xv. The detailed modalities for payment to the outsourced agency for different types of tests will be intimated subsequently along with MoU conditionalities.
xvi. Expenditure towards effective implementation of free diagnostic services may be done as per the guidelines issued from time to time under NHM, Nidaan, XV-FC etc.